Stored and Transported to maintain Therapeutic values






Stored and Transported For Therapeutic Values
Once essential oils and hydrosols are produced, they must be treated with care and must remain in their perfect state until the moment they are used. It sounds easy, but this is the stage at which much of the tampering actually takes place. Essential oils are frequently adulterated, fractionated, or boosted by real or synthetic chemical isolates to "improve" odor or simply increase volume and therefore profit.
Suffice it to say that even buying direct from distillers is not without pitfalls, and the large distributors and brokers of essential oils maybe many hands down the line from a honest producer. It is the responsibility of the individual to train their nose, study their botany, read the labels, know their suppliers, and truly understand their oils to determine and ensure that what they use are in fact real, authentic, whole, unaltered therapeutic essential oils.
Hydrosols, even more than essential oils, require great care after production. As they do not contain many of the antibacterial and non-water-soluble chemicals we find in oils, they have less natural preservative inherent in their makeup. Many of the distillers who keep their hydrosols instead of letting them run down the drain or into the field simply pour the waters into containers and put them on the shelf. The waters are not filtered, nor are the containers sterilized before filling hydrosols come of the still quite slowly, and it can take many hours to fill a receptacle with hydrosol.
During this time anything from bits of plant material to dust and insects can get into the vat. One company that sells over two tons of rose hydrosol per year recently told me they have even found gravel in their hydrosols during filtering. What of adulterants? We know about adulteration of oils, but who would adulterate water? Unfortunately the practice is not uncommon. Water is the most obvious adulterant used, since we're talking about water anyway, but diluting a hydrosol with plain or distilled water not only dilutes its effects but drastically shortens its life span.
Alcohol is another common adulterant of hydrosols. Alcohol, like water is undetectable by smell if it is low-enough concentrations, and it increases volume as well. But alcohol does not cost money and is mainly added as a preservative, greatly increasing a hydrosols shelf life by killing organisms and bacteria that may be present.
In countries o0f the European Union, hydrosols that are sold for cosmetic/aesthetic purposes must now by law contain alcohol, a minimum of 12 per cent volume. The fact that this renders the hydrosol almost useless for cosmetic purposes, since true hydrosols are alcohol free options for skin care, did not seem to influence the lawmakers .
However, the alcohol has been effective in preventing contamination and lengthening shelf life, even allowing hydrosols to be safely kept at room temperature in retail stores with no worry of spoilage-but more on this later.
Reference: Hydrosols The New Aromatherapy : Suzanne Catty
Economics Of Distillation







Economics Of Distillation
One of the ways a distiller can help offset some of the increased costs of therapeutic distillation is through the marketing of hydrosols. If the waters are not cohobated, distillation produces a lot of hydrosol - hundreds of litres, actually. Although not every drop produced throughout the distillation run is of therapeutic value, somewhere between twenty and thirty percent of the output can be used from an average large still.
Although each litre only sells for a few dollars, every twenty litres per run times the multiples f runs can amount to a reasonable sum of money added to the value of the essential oils. The distillers I work with have told me that selling their hydrosols has made a positive difference in their yearly incomes, and they consider hydrosols a coproduct, not a by-product, of the distillation process.
Essential oils produced by therapeutic distillation are expensive: are extremely labour intensive to produce: require the eye, hand, and heart of an artist combined with the mind of a scientist: and do not produce huge profits for the producers.
However, they not only offer us healing properties but can help us commune with and access the actual "life force" and knowledge of the plant, and on a larger scale they connect us with the planet and each other in new and healing ways. Spending more to purchase these oils is an easy choice when you look at it like this.
Reference: Hydrosols The Next Aromatherapy : Suzanne Catty
Distilled or Extracted Specifically For Therapeutic Use - 3







Distilled Or Extracted Specifically For Therapeutic Use - 3
As one of the students remarked afterward, " I thought I understood the work involved , but this changes everything. I'll remember this for the rest of my life!" And I'm sure she will. Many tabletop distillers are now available, and both science companies and aromatherapy companies offer distillation units for sale for the home experimenter. Even the Gaggia coffee company makes a still; it looks like a fancy cappuccino machine. In large-scale distillation, the steam is usually created in an outside source, although ancient designs and home stills usually boil the water for steam in the same pot in which you place the plant material. Water boils at one hundred degrees Celsius and at this point changes phase to become a gas, or steam. if the water is placed under pressure, the boiling temperature can be changed, similar to the way that altitude can effect the boiling point.
In therapeutic distillation the boiling point must be kept at one hundred degrees Celsius so the steam generator and still can operate under only atmospheric pressure. In industrial distillation the steam is generally superheated meaning that its boiling point is raised under pressure. This has two main functions: First, the hotter steam extracts the volatile components from the plant material more quickly, and second superheated steam moves more quickly, and second, superheated steam moves more rapidly and under pressure can literally be injected into the stills at an accelerated rate, vastly different from the gentle trickle preferred for aromatherapy products. Thus industrial distillation is shorter, more cost-effective, and concerned with the maximum output for the minimum cost.
Another practice that is more common in industrial distillation, although it has its place in therapeutic distillation as well, is cohobation. Cohobation means simply that the hydrosol or condensed water that comes off the still is recycled. The receiving vessel, called a Florentine flask, has an outflow that takes the water back to the steam generator, where it is reheated and passed through the plant material again. Cohobating the hydrosol means that any microdrops of essential oil that were dispersed in the water have an opportunity to coalesce into drops big enough to seperate into oil, thus improving the overall oil yield of the distillation. Water, which as we know is a precious commodity, is saved and the cost of production further lowered.
In therapeutic distillation, cohobation is used in distilling rose, where many of the four hundred-plus chemical components are highly water-soluble and the yield of the rose so low that every drop is extremely valuable. Uncohobated rose oil is missing many of the plant's important therapeutic and aromatic compounds because they stay in the water, and therefore it has little value and is never seen. Home distillation units frequently utilize cohobation because the tiny amount of plant material yields so little oil that the extra drops that cohobating can achieve make the process worthwhile. The downside of cohobation is that the resulting hydrosol is nearly useless from a therapeutic point of view, as it contains no dissolved essential oil, and the repeated heating of the water also seems to damage the water-soluble components in the hydrosol. But it still smells nice and is fun to play with.
Industrial distillation is perfectly acceptable when the resulting oil is to be used only for the extraction of specific compounds. The mono- terpene pinene in some pine oils is extracted for use in artificial apple flavours, scareol from clary sage is in cigarettes , and the camphor in corn mint is used in cold remedies and liniments. However, the extreme heat and pressure of the steam have damaging effects on some of the chemical constituents in the plant material and can " burn" the plant, giving an off flavor or odor to the oil as a whole. Also, essential oils are volatile, so excess heat frequently "boils off" some of the delicate high notes that are desirable in aromatherapy.
Let's look at the production of lavender oil for a minute. Most of the lavender commercially grown in France and other countries is actually a hybrid lavender more properly called lavandin. There are many lavandins: Lavendula hybrida x abrialis, x reydovan, x grosso, to name a few. These are the plants that you see in the stunning photographs of the lavender fields of Provence. Lavandin produces a much higher yield of essential oil than does true lavender (Lavandula officinalis, L. vera or L. angustifolia) usually three to four times the amount. But let's assume that we have true lavender plants to fill our still; what would we need to do to produce the most therapeutic oil possible?
If our still holds between 170 and 200 kilograms and we let it run for one hour, we would get approximately 1 kilogram of lavender oil. This would be true lavender oil, and if it was produced from organic plant material it could honestly be marked as "certified organic lavender essential oil". This is what many people are buying. However analysis of this oil would reveal that it likely did not contain the full range of the therapeutic chemicals that we want for aromatherapy. Most notably the coumarins would be missing.
Coumarins are one of the main sedative components of lavender oil and are produced only after the first eighty to ninety minutes of distillation. Back to the still. If we leave the distillation to continue after that first hour, for say, another two or three hours, we will have a total output of approximately 1.1 to 1.2 Kilograms. The first hour yields a kilogram and the next two hours one hundred or two hundred grams, an increase of only 10 to 20 percent; it's hardly worth it...or is it? If we analyze the oil at the end of the three -hour run, we will find that the coumarin content of the oil is now around 0.25 to 0.3 percent. A tiny amount, but it is enough to impart the renowned sedative property to lavender oil.
From a therapeutic point of view we must expect our oil to be complete and therefore contain this tiny percent of coumarins, but from a financial point of view we are virtually tripling the cost of the distillations, since the difference in overall yield is so minute.
Reference: Hydrosols The Next Aromatherapy / Suzanne Catty
Distilled or Extracted Specifically For Therapeutic Use - 2






Distilled or Extracted Specifically For Therapeutic Use - 2
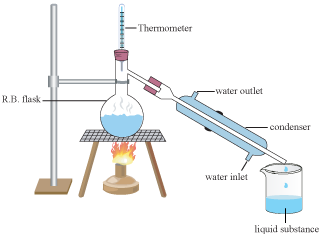
The condenser is cooled by running water that flows from the bottom to the top, surrounding the internal pipe with cold water, thus cooling the steam and converting it back into water and oil. That water is now a hydrosol. Most essential oils are lighter than water and will float on the surface of the hydrosol as it collects in the receiving vessel.
For this reason distillers use a Florentine flask to collect the output from the still. This specially designed container has two outflows . One drains the hydrosol from the bottom of the flask, allowing it to flow out and be collected ( which also serves to prevent the flask from overflowing ) and leaving the oil inside. The second outflow is near the top of the flask and is used only after the distillation is complete, when the oil is decanted through this pipe, leaving most of the remaining hydrosol in the Florentine.
Because distillation produces many times more hydrosol than oil, it is important that the two can be separated during the process. You couldn't make a Florentine large enough for all the water.This allows distillers to collect only the most therapeutic portion of the hydrosol ouput, usually the first 30 percent of a distillation run, although this varies from plant to plant. The hydrosol produced near the end of the run has little therapeutic value, as most of the components coming over by this point are too lipophilic to remain in the water.
If distillers add the end product to the more potent early hydrosol, the waters are diluted, reducing both their aromas and their properties. Other parameters relevant to collecting hydrosols can be found in a future chapter.In Hydro-distillation the plant material and water are combined in the still and the whole thing is then brought to a boil. The hot water draws out the oils, just as steam does and it is carried to the condenser and cooled into hydrosol and oil. This method is one of the oldest and has both benefits and drawbacks. First, it takes a huge amount of heat energy to bring a still-size volume of water and plant to a boil.
Just think how much longer it takes to boil. Just think how much longer it takes to boil a pot of potatoes than a kettle of water. Modern-day uses of this method are most current in countries where fire is used as the heat source or where electricity or gas are inexpensive. It is also the method used in India for the production of certain attars and ruhs, (the Indian name for hydro-distilled oils), notably from the flowers like tuberose, jasmine, rose and lotus. For attars, the receiving vessel contains sandalwood oil, which becomes infused with the flower essence; in ruhs no sandalwood oil is used.
Those who distill by this method say that it produces a finer, more complete product, as hot water is cooler than steam and shocks the plant material less. Also, there is less conversation of alcohols to esters in the chemical makeup of the oil, and certain other fragile and highly odiferous molecules may also be better retained.
I have had many hydrosols produced by hydro-distillation and can say that for the seed hydrosols, as well as some root and bark ones, this method does indeed seem to produce a richer hydrosol product but a much lower yield. With these tougher materials, the still can be filled with water and plant, then left to "stew" for twenty-four hours or more before distillation is begun. This seems to allow more before distillation is begun. This seems to allow more of the water-soluble components to be extracted. For other plant material there seems to be much less difference in the products of therapeutic steam-versus water-distilling. There is still no agreement as to whether the life span is shorter or longer for hydro-distilled hydrolates versus the steam-distilled version.
Hydro-diffusion is an odd, if interesting, approach. In this case the steam inlet is at the top, above the plant material, and the outflow to the condenser is below. When you consider that steam is prone to flow up, not down, you can see what I mean by odd! The shape and dimensions of the still are different from those used in ffffsteam or hydro-distillation, being wider than tall, in this case.
This method has proved popular in countries where water is in short supply, as the steam is already partially cooled by its travel through the plants, so it requires less cooling in the condenser to be turned back into water and oil. Bear in mind that it takes a lot of water to keep a condenser cool, and many distillers heat their swimming pools, greenhouses, or homes by recycling this water. In hydro-diffusion, as in steam-distillation, the steam is created in an outside source, requiring less energy to produce than in hydro-distillation.
Many countries in Africa are using hydro-diffusion, and their water scarcities are as legendary as their abundance of therapeutic plant material. so I am glad this method is available.The hydrosols and oils produced by this method are every bit as lovely as those produced by steam or water.
Distillation is such an art that you can count on your fingers and toes the number of truly great aromatherapy distillers in the world. They account for around 2 percent of the total global production of essential oils made each year. All the rest, whether organic or industrial, fall short by comparison. For this reason, and for the sheer fun of it, many aromatherapists today want to try distilling themselves . We are all alchemists at heart, and distilling on our won provides a much deeper understanding of the plants and the oils and hydrosols they produce.
I take all my students to an aromatherapy distillery as part of their training,: it is the only way to truly understand the level of work, commitment, and love required to produce our precious aromatics. This year they spent more than half a day in the forest, surrounded by black flies and mosquitoes, gathering just four hundred pounds of larch branches, tying them in bundles, and hiking them back out of the woods, fording streams and ditches.
This four hundred pounds only half filled the still, and after nearly six hours of distillation we produced just 270 milliliters of the exquisite pale green oil.
Read More...Distilled or Extracted Specifically For Therapeutic Use - 3
Reference: Hydrosols The Next Aromatherapy / Suzanne Catty
Articles - Most Read
- Home
- What are Hydrosols
- What are Hydrosols-2
- The Monographs
- How to Make a Hydrosol
- Distilled or Extracted Specifically For Therapeutic Use - 3
- Table of Common Latin Names and pH Values - F - O
- Kurt Schnaubelt
- What isn't a Hydrosol?
- Table of Common Latin Names and pH Values - P - S
- Wholly Water!
- Blue Babies
- Supply and Demands
- Mature Skin
- Recipes Alpha F
- Hydrosols In The Marketplace
- Hemorrhoids
- Nelly GrosJean
- Chemicals: Friends or Foes?
- Water as Medicine
- The Educated Consumer
- Influences
- Genitically Modified Plants
- Water Quality
Articles-latest
- Daucus carota/Wild Carrot Seed - pH 3.8-4.0
- Cupressus sempervirens/ Cypress-pH3.5-3.7
- Coriandrum sativum/Coriander Herb-and-Seed
- Comptonia peregrinal/Sweet Fern- pH 3.8
- Citrus clementine (fe) Clementine Petitgrain- pH 4.3-4.4
- Citrus aurantium var. amara (flos) /Neroli Orange Blossom-pH3.8-4.5
- Cistus ladaniferus/Rock Rose-pH 2.9-3.1
- Cinnamomum zeylanicum (ec) Cinnamon Bark-pH3.3
- Chamaemelum nobile/Roman Chamomile - pH 3.0-3,3
- Centaurea cyanus/Cornflower/Bachelor's Button-pH 4.7-5.0
- Cedrus atlantical/Cedarwood/Atlas Cedar-pH 4.1- 4.2
- Hydrosols -The PH - Anomalies
- Hydrosols- Establishing Shelf Life and Stability
- Boswellia carterii/FRANKINCENSE
- Asarum canadense/ Wild Ginger/Canadian Ginger
- Artemesia vulgaris / Artemesia
- ARTEMESIA DRACUNCULUS - TARRAGON
- Angelica archangelica / Angelica Root - Hydrosols
- The Key, or More Correctly, the pH - 2 - Hydrosols
- The Key, or More Correctly, the pH-Hydrosols
- The Hard pHacts - Hydrosols
- Calamus Root/Sweet Flag - ACORUS CALAMUS
- Yarrow - Achillea millefolium - Hydrosols
- Balsam Fir - Abies balsamea - Hydrosols

